Future Experimental Directions
Many different mutations in the dystrophin gene have been shown to result in a DMD phenotype. One of the most common of these are frameshift mutations, which are commonly located in the spectrin repeat domains of the dystrophin gene. These frameshift mutations result in completely non-functional protein downstream of the mutation. Similar mutations resulting in premature termination codons (also often located in the spectrin repeat domains) have been shown to be rescuable by certain small molecules in high-throughput screens. Such small molecules promote readthrough of the premature stop codons in a fraction of dystrophin translation events that is sufficient to rescue near wildtype functional dystrophin protein expression. Because of these findings, and the prevalence of frameshift mutations in DMD patients, I propose that a chemical genetic screen be performed to identify compounds that may promote slippage or readthrough of frameshift-causing mutations.
I hypothesize that there are one or more small molecules that may result in translational readthrough of frameshift-inducing mutations and thus are capable of rescuing the wildtype phenotype in DMD individuals with the specific types of mutations.
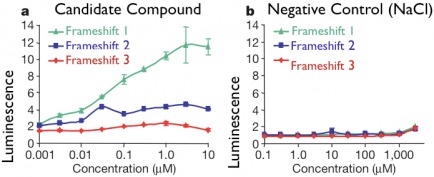
I propose that first a high throughput chemical genetic screen be performed where thousands (or more) small molecules are tested for ability to rescue functional dystrophin translation in at least three different frameshift mutant strains. I would first use an in vitro assay with translation-competent extract and the dystrophin strains tagged with GFP at the N-terminus. An experimental setup such as this will provide for the high-throughput experimentation required to test a large number of compounds. I would expect that some potential frameshift-suppressing chemicals will be found, and will give rise to increased GFP luminescence (indicating the presence of functional dystrophin) compared to a negative control. Such results are summarized in the figure below.
I hypothesize that there are one or more small molecules that may result in translational readthrough of frameshift-inducing mutations and thus are capable of rescuing the wildtype phenotype in DMD individuals with the specific types of mutations.
I propose that first a high throughput chemical genetic screen be performed where thousands (or more) small molecules are tested for ability to rescue functional dystrophin translation in at least three different frameshift mutant strains. I would first use an in vitro assay with translation-competent extract and the dystrophin strains tagged with GFP at the N-terminus. An experimental setup such as this will provide for the high-throughput experimentation required to test a large number of compounds. I would expect that some potential frameshift-suppressing chemicals will be found, and will give rise to increased GFP luminescence (indicating the presence of functional dystrophin) compared to a negative control. Such results are summarized in the figure below.
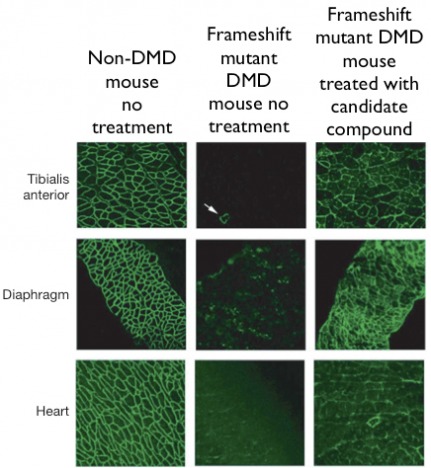
After the high throughput screen is performed, it would be appropriate to assay for the same activity of the candidate molecules in vivo. This would be most appropriately done in mice, due to their very similar form of dystrophin (as described on the homology page), their similar physiology to humans, and the well-characterized dystrophin mutant strains. To assay for the frameshift-silencing ability of the candidate molecules, each compound would be tested on three strains of frameshift mutant mice (as in the in vitro assay) and striated muscle tissue samples would be observed for the presence of functional dystrophin by immunohistochemistry. An expected result for a candidate frameshift-suppressing compound is shown below.
Of course, further testing for potential toxicity and mutagenicity of the compounds would be required, but this proof-of-concept experiment would provide a major step forward towards personalized therapies for DMD-affected individuals with hope to them an extended lifespan with a drastically improved quality of life.
References
Welch, E., E. Barton, J. Zhuo, Y. Tomizawa, W. Friesen, and P. Trifillis. "PTC124 Targets Genetic Disorders Caused by Nonsense Mutations."Nature 447 (2007): 87-93. Web. 10 Apr. 2010.
"SMART: DMD Domain Annotation." SMART: Main Page. Web. 22 Feb. 2010. <http://smart.embl.de/smart/do_annotation.pl?DOMAIN=SM00150>.
"SMART: DMD Domain Annotation." SMART: Main Page. Web. 22 Feb. 2010. <http://smart.embl.de/smart/do_annotation.pl?DOMAIN=SM00150>.
This site was created by Anthony Parendo for an undergraduate Genetics 677 course at the University of Wisconsin-Madison.